from Textile Artists' Newsletter [TAN] Vol. III, No. 3 from 1982
Textile Artists' Newsletter, TAN, was published from Straw Into Gold in Oakland and later Berkeley, California in the early 1980s. Susan C. Druding was the Publisher/Editor and articles were written by her and many other textile artists. We are scanning old issues and putting the information on the Straw.com web site. Much of it is still very useful still.
All TAN articles reproduced on this site are copyright 2000 by Susan C. Druding - not to be reproduced or copied in print or electronic media without permission. You may, of course, print out a copy for your own use. Thank you.
Fiber Reactive Dye recipes can be found on this page as well.
Fiber Reactive Dyes and Cibacron F in particular |
This issue of TAN, Vol III, no. 3 was especially devoted to explaining fiber reactive dyes and Cibacron F dye. There were several articles in this issue, all written by Susan Druding. They are all here on this single page showing each article headline.
Remember, as you read, that these articles were written in 1982 and there have been changes and new dyes introduced since then.
A Definition
The official definition of a "fiber reactive dye" is provided by Rys and Zollinger in chapter VII of their book, The Theory of Coloration of Textiles (1975) from the Dyers Company Publications Trust, England. A fiber reactive dye "is a coloured compound which has a suitable group capable of forming a covalent bond between a carbon atom of the dye ion or molecule and an oxygen, nitrogen, or sulphur atom of a hydroxy, an amino or a mercapto group
respectively of the substrate." They point out that the definition excludes mordant dyes and 1: 1 chromium azo dye complexes which, in dyeing protein fibers may form covalent bonds between metal ion and nucleophilic groups of the fiber.
What all this means is that a fiber reactive dye reacts to form a true bond (not just a plus or minus charge attraction or an entrapment in the fiber) with the fiber involved. In the case of cellulose the bond is with the hydroxyl (-OH) groups present in vast numbers on the cellulose molecule and in the case of protein fibers with the amino (-NH3 ) group present on the protein molecule.
New Discoveries
Fiber Reactive Additions Enrich Artists' Palette
This article began as an explanation of CIBA- Geigy's cold water fiber reactive dye, Cibacron F. But it grew into a cluster of articles and information on fiber reactive dyes. There is still so much confusion among home and studio dyers about the various types of fiber reactive dyes, that it seemed appropriate to try to clear up some of those misunderstandings.
Procion MX
Procion MX dyes are the fiber reactive dyes most used by dyers, batikers, printers of cotton and rayon fabrics, and for craft and school use. Their ease of use comes from the fact that they have a very high reactivity due to their chemical structure.
This reactivity allows them to be set or fixed on the fiber without the need for simmering or complicated steaming procedures. The water temperatures required for their use are in the 105 to 140 degree F range (that is, hot tap water).
Confusion In Names
Confusion comes from the fact that many users of Procion MX do not know that they are using it. This is because there have been, and still are, a wide variety of packaged dyes sold under various trade names, which are, in fact, Procion MX. Some of these include: Dylon, Fibrec, Linda's Dye, Hi-Dye, Putnam's Fiber Reactive (some of these are no longer being packaged), and others. A white fixative powder (washing soda or sal soda) Is sometimes included and common salt (sodium chloride) is required. Because of this proliferation of names, which were all really Procion MX, some textbooks on batik or craft dyeing go so far as to state that "every fiber reactive dye in the world is Procion."
Cibacron F Introduced
|
|
Being "the only fiber reactive dye in the world" would certainly please the sales force of Procion's manufacturer, ICI, if it were true. But such is not the case. Although it was true a few years ago that only the Procion MX series was easily available for home or studio use, at least 20 manufacturers make some form of fiber reactive dye.
In 1979, there finally came a new, "cold water" fiber reactive to compete In the industrial market with Procion MX, which until then had been the only highly reactive dye available. The new dye Is called Cibacron F and is a product of CIBA-Geigy Corp. of Basle, Switzerland. Cibacron F dyes are marketed for craftspeople and artists under the name "Reactive Dye" by Spectrum Dyestuffs of Berkeley, CA. [note: now in year 2000 Spectrum Dyes is no longer in business.]
Cibacron F dyes can be mixed with Procion MX series even though take up rates will vary. Since the Cibacron F color series does not include a turquoise, many dyers use the turquoise from the Procion MX series.
Its ability to "cold batch," efficiently, that is to be set without heat or steam, means that it is a welcome addition to the palettes of weavers who warp paint, and printers and fabric painters who work with limited equipment. Silk surface designers appreciate the ease of application and rich colors on silk without steaming.
Now that both Procion MX and Cibacron F dyes are available, artists have a choice in cold water dyes and many have found that the increase in the palette of colors from which they can choose to work has given them added possibilities.
By Susan C. Druding
Fiber Reactive Dyes: The History
Wide Range of Dyes Follows 1st Success
Only one type of fiber reactive dye, Procion, is familiar to most art and craft dyers. Actually, there are more than 20 manufacturers of various types of reactive dyes in at least 12 different chemical systems.
As everyone knows who has read the numerous articles or heard lectures about the recent history of dyestuffs, ICI (Imperial Chemicals Industries) introduced Procion to the world in 1956.
This was, indeed, a very significant breakthrough. What no one ever hears about, of course, is that there was a "runner up" in the race to be first. That was CIBA Ltd. of Basle, Switzerland. From available information it seems that neither of them was aware that a truly marketable dye was so close.
It must have been quite a shock in Switzerland the day ICI made its announcement. CIBA had been doing research since the 1930s on the class of chemical dye compounds which resulted in the fiber reactive dyes, but ICI was the first to make the official product announcement and to file patents.
All was not lost for CIBA-Geigy, however, for it had a strong patent position in research with the same chemical compounds for its Chlorantine Direct Dyes. So while neither CIBA-Geigy (nor any other manufacturer) could duplicate Procion MX dyes CIBA-Geigy did come out with a series of fiber reactive dyes called Cibacron A that competed with ICI's Procion H series. Both these systems came out in 1957, the year after ICI introduced Procion MX dyes. ( CIBA Review, No. 120 for June, 1957 devotes the entire issue to those new Cibacrons Many libraries have these Reviews.)
The huge success of ICl's first Procion dye brought about a real revolution in the dye industry. All the major dye manufacturers began research programs to develop other types of reactive dyes.
Before 1956 few chemists believed that a true fiber reactive dye that could be used commercially would ever be developed. This disbelief quickly changed to furious research and a astonishing series of successes was achieved in short time.
Once the general principles had been established a great amount of research work quickly produced a variety of other systems. Some of the other reactive dyes developed during this period (although seldom used by crafts people) are: Bayer's Levafix, Geigy's Reactone, Sandoz's Drimarene, DuPont's Cavalite, BASF's Primazin, and Hoechst's Remazol. None of these however, used temperatures as low as those used for Procion MX dyes and craftspeople stayed with the MX series.
Years passed and research continued, but most dyers assumed that the cellulose fiber reactive field had settled down to a regular group of contenders. Who can say exactly why CIBA-Geigy decided to add the first competitor to the field of highly reactive, "cold" water dyes? For a certainty the energy shortages and need to conserve power (as cold water dyes do) had something to do with it. Also, Procion MX series has the problem of being too reactive in many circumstances. The reactivity is so high that solutions and print pastes have very short life before hydrolysis begins to make the dye strength drop.
With the advances in dye chemistry the chemists in Switzerland were able to design a new cold water dye that was able to react more slowly to conserve dye life, yet still be reactive enough that it could be used as a "cold water" dye. Perhaps the challenge of making a dye work that had fluorine rather than chlorine as its reactive group was a part of it. As recently as 1971, a dye chemistry book was published which stated that "fluorine has been mentioned in many patent examples, but is not known to have been used commercially."
Certainly, many market studies preceded such a major decision as launching a new dye group. But, one cannot help but think that after more than 20 years there wasn't still the feeling that ClBA had to eventually, someday come out with a challenge to that very first ICI fiber reactive dye that caused such a stir in 1956, just slightly more than a year before the first Cibacrons were introduced. Perhaps someday a CIBA-Geigy chemist will write his memoirs about this very interesting period in the history of dye chemistry.
In the fall of 1978, under a good deal of industrial secrecy, CIBA-Geigy dye chemists were flown to a special seminar in Basle, Switzerland. The seminars, conducted in several languages, explained all about a new dye: Cibacron F. The actual chemical formulas are still not public knowledge, but the dye chemistry is based on fluorine. Fluorine is related closely to chlorine, but is more reactive. Apparently, the use of fluorine leaves more "space to play" on the molecule. This means that solubility, exhaust rates, and many other dye properties could be made more sensitive than they could in the then current chlorine (chloro-) based types of dyes.
Advertisements for the new dye began to appear in the professional journals, the logo that of a hand with a watch to emphasize the speed of the reaction.
Since its introduction to the art and craft world in 1980 (under the Spectrum Dye name "Reactive Dye",), Cibacron F has become very popular. Artists like the many features, especially its excellent leveling capabilities (non-streakiness), the matched capabilities of all the colors in the range to give even color all through dyeing, its excellent penetration of the fiber and its extended life due to its slightly lower reactivity.
Fiber Reactive Dyes: The Chemistry
Most Reactives Built On Similar Structure
Various types of reactive dyes available for commercial use differ widely in their reactivity for cellulose fibers. They range from very low reactivity to highly reactive (such as Cibacron F and Procion MX). The difference between the lowest and highest reactivity is a factor of 1,000 times.
All but one of the reactive dyes are built on a similar structure (Remazol Dye from Hoechst is the exception). This structure consists of (1) a chromophore (the color-bearing group), (2) a reactive group (usually a heterocyclic carbon-nitrogen ring system), and (3) a "leaving group" which is part of the carbon-nitrogen group which is generally a halogen compound (chlorine family).
This "leaving group" splits off during the reaction with the fiber and is the point at which the bond is formed. The level of reactivity of dyestuff is mainly dependent on the reactive group and on the "leaving group".
In the earlier fiber reactive dyes (such as the Procions and early Cibacrons) the "leaving groups" were always chlorine, but later it was found that other groups could impart even higher reactivity. These groups could be attached to the dye molecules and affect such things as fixation rates, solubility, substantivity (the attraction of the dye for the fiber), and build-up (the depth of color possible).
These early fiber reactive dyes based on chlorine chemistry were known as cholortriazinyl dyes. The reactive group was a triazinyl ring (a six-sided ring with three nitrogens); if it had one chlorine built into it the dye was called a monochlorotriazinyl dye (see Fig 1); if it had two chlorines the dye was more reactive and called a dichlorotriazinyl dye. (See Fig. 2)
In Fig. 1 is a typical monochlorotriazinyl dye and note that the chemical structure, and thus the dye itself, is identical for this Procion H Scarlet H-R and Cibacron E dye Scarlet RP. Not all the dyes in the two ranges are identical, but there are several overlaps.
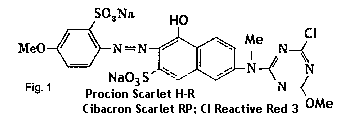 |
|
fig. 1 - a monochlorotriazinyl dye molecule |
The Procion H series and Cibracron E series, both introduced in 1957, were monochlorotriazinyl dyes. These are less reactive than the Procion MX series and require higher temperatures, more alkali, and longer fixation times. They have a higher fixation level so less dye is lost, but they cannot be used at the low temperatures which make the "cold water" types more attractive to textile and fiber artists.
ICI's first Procion MX dyes were dichlorotriazinyl dyes. They are the most highly reactive because of their two chlorine groups.
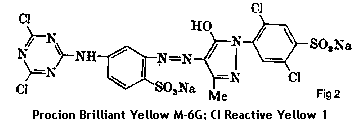 |
|
fig 2. - a typical dichlorotriaznyl dye |
The new Cibacron F dyes are a monofluorotriazinyl dye. Since fluorine is more reactive than chlorine, it makes them more reactive than the monochloro- dyes, but slightly less reactive than the dichloro- dyes. Not only was the "leaving group" changed to fluorine, but important changes were made in the other groups which affect the behavior of the dye in the way in which it levels, the speed of take up, and other features. The chromophores (color components) were carefully chosen for the best light fastness and high tinctorial strength.
Cibacron F
Careful Planning Leads to Better Dye
By Susan C. Druding
The Swiss chemists at CIBA-Geigy first "designed" Cibacron F fiber reactive dye on paper. Then they spent years developing a dyestuff that fit perfectly the properties they wanted it to have.
We learned this bit of background Information during a phone talk with a CIBA-Geigy product engineer. We asked what he felt were the most important features of Cibacron F that would distinguish it from the older cold water series, Procion MX. Following are the results of that discussion.
Efficient Penetration
The chemists designed the dye molecule chemically to be efficient at penetrating fibers prior to fixation. This means the dye diffuses quickly into the fiber, and yarns are not left with undyed centers.
This is an important feature in a cold water dye since the lower temperature makes the fibers harder to penetrate. The fibers, of course, must be properly cleaned and wet first.
Wash Off Much Easier
The chemists adjusted the attraction of the dye to the fiber (called substantivity) to be great enough to be attracted to the fiber, but not so great that the unfixed dye is hard to wash off after dyeing is completed. With proper wash off, this dye is much easier to use.
Levelling Improved
Cibacron F shows a marked improvement In levelling characteristics (its ability to dye evenly). During the first stage of dyeing, when the dye Is not attached to the fiber. It is much better at spreading itself evenly throughout the material.
Therefore, when soda is added later to fix the dye, streaking is not a problem.
Less Dye for Deep Shades
Deep shades are possible because of high tinctorial strength and good color build up. This means deeper, more intense shades are possible with less dye than necessary with Procion MX, making Cibacron F more efficient.
True Jet Black Possible
Cibacron F dyes can be mixed to yield a true jet black on rayon and cotton. A black has not been possible with the Procion MX series.
Similar Exhaust Rates
The most impressive feat of the Swiss chemists is the mutual compatibility of the Cibacron F dyes. They exhaust at very similar rates. Curves plotted for each dye color by temperature and time can be laid over one another with practically no variation. This is important for several reasons:
All Cibacron F dyes hydrolyze (react with water and become "dead") at the same rate. Printers know that because colors in Procion MX mixtures hydrolyze at different rates, the color of the print paste will shift, in other words actually change, over a 15 to 60 minute period. With Cibacron F mixtures, even if the dye paste begins to lose strength after 90 minutes, it will lose it evenly in all colors and the shade will not alter.
Dyers who like to judge color mixes visually while dyeing appreciate knowing that the Cibacron F shade seen after 10 minutes is the same, but lighter version, it will be after 20 more minutes. Many other dye systems make this visual check impossible because different colors "take" at different rates by moving on to the fibers earlier or later than the others.
This feature also makes it possible to get gradations in intensity of color mixes in the same dye bath without a color shift by adding fibers to the pot in steps. The even rate of take up means one or more colors won't be "missing" when new fibers are added.
Wider Temperature Range
Due to its fluorine chemical structure, Cibacron F, while still highly reactive, is slightly less reactive than the Procion MX series. This has several advantages:
For yarn dyeing Cibacron F works under a wider temperature range than Procion MX. It may be used as high as 175 degrees F without damage. Recommended temperature range is 105 to 120 F (40 to 50 C). If used below 105 F, dyeing time should be extended and a lower yield expected. Procion MX should not be heated over 140 degrees F.
For surface application, Cibacron F is more successful in the cold pad batch technique (setting the print without heat or steam by leaving it under a wrapper of plastic for 24 to 48 hours) than Procion MX. Procion MX, because of its very high reactivity, begins to hydrolyze within a few minutes after it is in contact with soda thus leaving less dye in the print paste available for reacting with the fabric. Cibacron F dyes remain stable much longer and give deeper shades with less dye in the cold batching.
Cibacron F dyes are not as sensitive to temperature and soda variations. This means there is no "burn out" problem. For those who are somewhat less than careful in measurements than they could be, there is always the likelihood of overdosing with soda and heat. Cibacron F dyes are more resistant to this than Procion MX.
Cibacron F liquid stock solutions (without soda) can be kept many weeks in the refrigerator with very little loss of strength due to their lower reactivity rate. Procion MX lasts less than half as long.
References:
Abrahart, E. N.; Dyes and Their Intermediates, Chemical Publishing Co., NY, 1977
Allen, R. L. M.; Colour Chemistry, Appleton-Century-Crofts, NY, 1971
C I BA Review No. 120, Special Number-Cibacron Dyes, June, 1957, CIBA Ltd., Basle Switzerland
"Cibacron F Dyes", sales booklet, CIBA-GeigyCorp., 1978, Switzerland
"Cibacron F dyes in the exhaust and cold pad-batch methods on cellulosic fibres," products brochures, CIBA-Geigy, 1978, Switzerland.
Siegrist, Dr. G.; "Optimising the reactive dyeing of cellulosic fibres," International Dyer, V.163 no. 9, May, 1980, Great Britain
"Procion Dyes for Exhaust Dyeing," technical bulletin, ICI America Inc., 1973, Wilmington
"Cibacron F" is a registered trademark of CIBA-Geigy Ltd. of Basle, Switzerland.
"Procion MX" and "Procion H" are registered trademarks of ICI (imperial Chemicals Industries) of Great Britain.
All TAN articles reproduced on this site are copyright �2000 by Susan C. Druding - not to be reproduced or copied in print or electronic media without permission. You may, of course, print out a copy for your own use. Thank you.
Straw Into Gold - Susan Druding
URL of this page: http://www.straw.com/tan/tan_fiberreactives.html